IMPORTANT SAFETY INFORMATION
XATMEP® (methotrexate) Oral Solution, 2.5 mg/mL
INDICATIONS:
XATMEP is a folate analog metabolic inhibitor indicated for the:
- Treatment of pediatric patients with acute lymphoblastic leukemia (ALL) as part of a
multi-phase, combination chemotherapy maintenance regimen.
- Management of pediatric patients with active polyarticular juvenile idiopathic arthritis (pJIA)
who have had an insufficient therapeutic response to, or are intolerant of, an adequate trial of
first-line therapy including full dose non-steroidal anti-inflammatory agents (NSAIDs).
WARNING: SEVERE TOXIC REACTIONS, INCLUDING EMBRYO-FETAL TOXICITY
See Full Prescribing Information for complete boxed warning.
- Methotrexate can cause severe or fatal toxicities. Monitor closely and modify dose or discontinue for the following toxicities: bone marrow suppression (5.1), infection (5.2), renal (5.3), gastrointestinal (5.4), hepatic (5.5), pulmonary (5.6), hypersensitivity and dermatologic (5.7).
- Methotrexate can cause embryo-fetal toxicity and fetal death. Use in polyarticular juvenile idiopathic arthritis is contraindicated in pregnancy (4). Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. Advise patients to use effective contraception during and after treatment with XATMEP (5.9, 8.1, 8.3).
A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device.
The recommended dose should be taken one time weekly, as directed. Mistaken daily use of the recommended dose has led to fatal toxicity.
ADDITIONAL IMPORTANT SAFETY INFORMATION:
CONTRAINDICATIONS
XATMEP is contraindicated in
- Pregnant patients with polyarticular juvenile idiopathic arthritis (pJIA)
- Known severe hypersensitivity to methotrexate or any of its components
Warnings and Precautions
Bone Marrow Suppression.XATMEP suppresses hematopoiesis and can cause severe and life-threatening pancytopenia, anemia, leukopenia, neutropenia, and thrombocytopenia.
Obtain blood counts at baseline and periodically during treatment. Monitor patients for possible clinical complications of bone marrow suppression. Provide supportive care and modify dose or discontinue XATMEP as needed.
Serious Infections. Patients treated with XATMEP are at increased risk for developing life-threatening or fatal bacterial, fungal, or viral infections including opportunistic infections such as Pneumocystis jiroveci pneumonia, invasive fungal infections, hepatitis B reactivation, tuberculosis primary infection or reactivation, and disseminated Herpes zoster and cytomegalovirus infections.
Monitor patients for the signs and symptoms of infection during and after treatment with XATMEP and treat promptly. Consider dose modification or discontinuation of XATMEP in patients who develop serious infections.
Renal Toxicity and Increased Toxicity with Renal Impairment. XATMEP can cause renal damage including acute renal failure. Monitor renal function to decrease the risk of renal injury and mitigate renal toxicity.
Consider administration of glucarpidase in patients with toxic plasma methotrexate concentrations (> 1 micromole per liter) and delayed clearance due to impaired renal function.
Gastrointestinal Toxicity. XATMEP can cause diarrhea, vomiting, stomatitis, hemorrhagic enteritis, and fatal intestinal perforation. Patients with peptic ulcer disease or ulcerative colitis are at a greater risk of developing severe gastrointestinal adverse reactions.
Interrupt or discontinue XATMEP and institute appropriate supportive care as needed.
Unexpectedly severe and fatal gastrointestinal toxicity can occur with concomitant administration of XATMEP (primarily at high dosage) and nonsteroidal anti-inflammatory drugs (NSAIDs).
Hepatic Toxicity. XATMEP can cause severe and potentially irreversible hepatotoxicity including fibrosis, cirrhosis, and fatal liver failure. Avoid use of XATMEP in patients with chronic liver disease.
Assess liver function prior to initiating XATMEP and monitor liver function tests during treatment. Interrupt or discontinue XATMEP as appropriate. Transient asymptomatic acute liver enzyme elevations are common and are not predictive of subsequent hepatic disease. Persistent abnormalities in liver function tests may precede appearance of fibrosis or cirrhosis.
Other risk factors for hepatotoxicity include alcoholism, obesity, diabetes, hyperlipidemia, previous significant exposure to liver toxins, history of liver disease, family history of inheritable liver disease, persistent abnormal liver chemistry findings, duration of therapy, and advanced age.
Pulmonary Toxicity. Methotrexate-induced pulmonary toxicity including acute or chronic interstitial pneumonitis and irreversible or fatal cases can occur at all dose levels. Monitor patients for signs of pulmonary toxicity and interrupt or discontinue XATMEP as appropriate.
Hypersensitivity and Dermatologic Reactions. Severe, including fatal, dermatologic reactions, such as toxic epidermal necrolysis, Stevens-Johnson syndrome, exfoliative dermatitis, skin necrosis, erythema multiforme, can occur with methotrexate. Discontinue XATMEP if severe dermatologic reactions occur.
Anaphylaxis can occur with methotrexate. If anaphylaxis or any other serious hypersensitivity reaction occurs, immediately discontinue methotrexate and institute appropriate therapy. Methotrexate is contraindicated for use in patients with a history of severe hypersensitivity.
Radiation dermatitis and sunburn may be “recalled” by the use of methotrexate.
Secondary Malignancies. Secondary malignancies can occur at all dose levels of methotrexate.
There have been instances of lymphoproliferative disease associated with low-dose oral methotrexate which have regressed completely following withdrawal of methotrexate without institution of antineoplastic therapy. Discontinue XATMEP first and institute appropriate treatment if the lymphoma does not regress.
Embryo-Fetal Toxicity. Based on published reports and methotrexate’s mechanism of action, methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman. In pregnant women with non-malignant diseases, methotrexate is contraindicated. Consider the benefits and risks of XATMEP and risks to the fetus when prescribing XATMEP to a pregnant patient with a neoplastic disease. Advise females of reproductive potential to use effective contraception during therapy and for 6 months after the final dose. Advise males of reproductive potential to use effective contraception during and for at least 3 months after the final methotrexate dose.
Ineffective Immunization and Risks Associated with Live Vaccines. Immunization may be ineffective when given during XATMEP therapy.
Immunization with live virus vaccines is not recommended. There have been reports of disseminated vaccinia infections after smallpox immunization in patients receiving methotrexate therapy.
Effects on Reproduction. Based on published reports, methotrexate can cause impairment of fertility, oligospermia, and menstrual dysfunction. It is not known if the infertility is reversible in affected patients. Discuss the risk of effects on reproduction with female and male patients.
Increased Toxicity Due to Third-Space Accumulation. Methotrexate can exit slowly from third-space accumulations resulting in prolonged terminal plasma half-life and toxicity. Evacuate significant third-space accumulations prior to methotrexate administration.
Soft Tissue and Bone Toxicity with Radiation Therapy. Concomitant radiation therapy increases the risk of soft tissue necrosis and osteonecrosis associated with methotrexate.
Laboratory Tests. Closely monitor patients undergoing XATMEP therapy so that toxic effects are detected promptly. In general, monitoring of the following parameters is recommended: hematology at least monthly, renal function and liver function every 1 to 2 months.
Increase monitoring frequency during initial dosing, dose changes, or during periods of increased risk of elevated methotrexate blood levels (e.g., dehydration).
Liver Function Tests
Transient liver function test abnormalities are observed frequently after methotrexate administration and are usually not cause for modification of methotrexate therapy. Persistent liver function test abnormalities, and/or depression of serum albumin may be indicators of serious liver toxicity and require evaluation.
Pulmonary Function Tests
Pulmonary function tests may be useful if methotrexate-induced lung disease is suspected, especially if baseline measurements are available.
Risk of Improper Dosing. Both the physician and pharmacist should emphasize to the patient that the recommended dose is taken one time weekly, as directed, and that mistaken daily use of the recommended dose has led to fatal toxicity.
Advise patients to measure XATMEP with an accurate milliliter measuring device. Inform patients that a household
teaspoon is not an accurate measuring device and could lead to overdosage, which can result in serious adverse reactions. Advise patients to ask their pharmacist to recommend an appropriate measuring device and for instructions for measuring the correct dose.
ADVERSE REACTIONS
Most common adverse reactions are ulcerative stomatitis, leukopenia, nausea, abdominal distress, and elevated liver function tests. Other frequently reported adverse reactions are malaise, fatigue, chills and fever, dizziness and decreased resistance to infection.
These are not all the possible side effects of XATMEP. Please see Full Prescribing Information for a full list.
DRUG INTERACTIONS
Oral Antibiotics: May increase hematologic and gastrointestinal toxicity. Monitor patients accordingly.
Nitrous Oxide: May increase the risk of toxicity.
NSAIDs, Aspirin, and Steroids: May elevate and prolong serum methotrexate levels and increase gastrointestinal toxicity. Monitor patients accordingly.
See Full Prescribing Information for Specific Drugs and Interactions.
USE IN SPECIFIC POPULATIONS
Lactation: Advise women not to breastfeed.
The Important Safety Information does not include all the information needed to use XATMEP safely and effectively. Please see accompanying Full Prescribing Information for XATMEP.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
XATMEP® is a trademark of Azurity Pharmaceuticals, Inc.
©2025 Azurity Pharmaceuticals, Inc.
PP-XAT-US-0111
References: 1. Xatmep [package insert]. Wilmington, MA: Azurity Pharmaceuticals, Inc.; 2020.
2. Chappell F. Medication adherence in children remains a challenge. Prescriber. 2015;26(12):31-34.
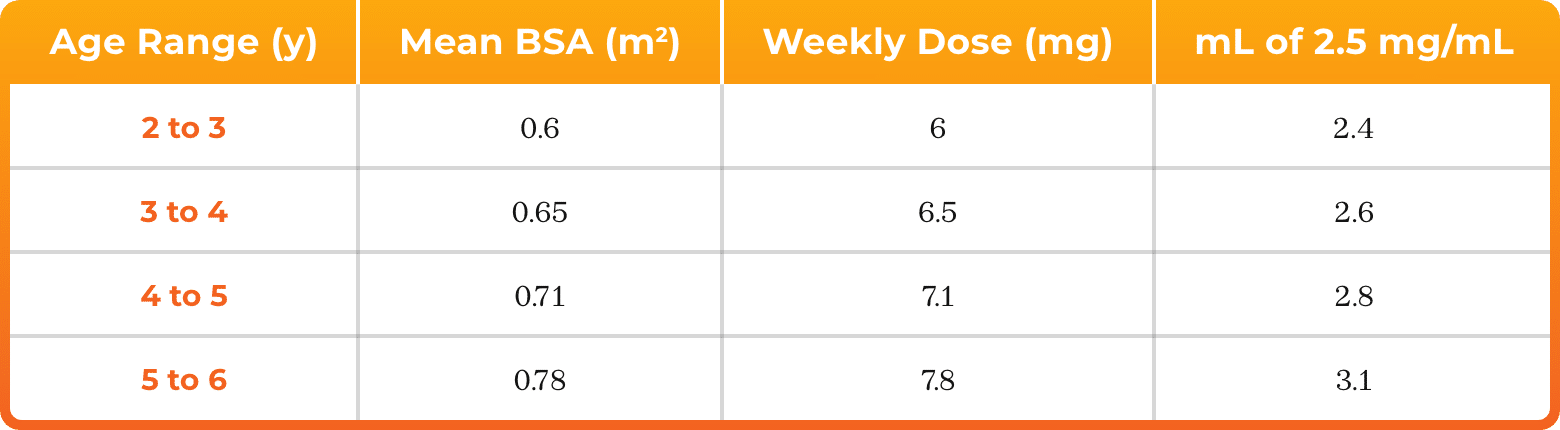
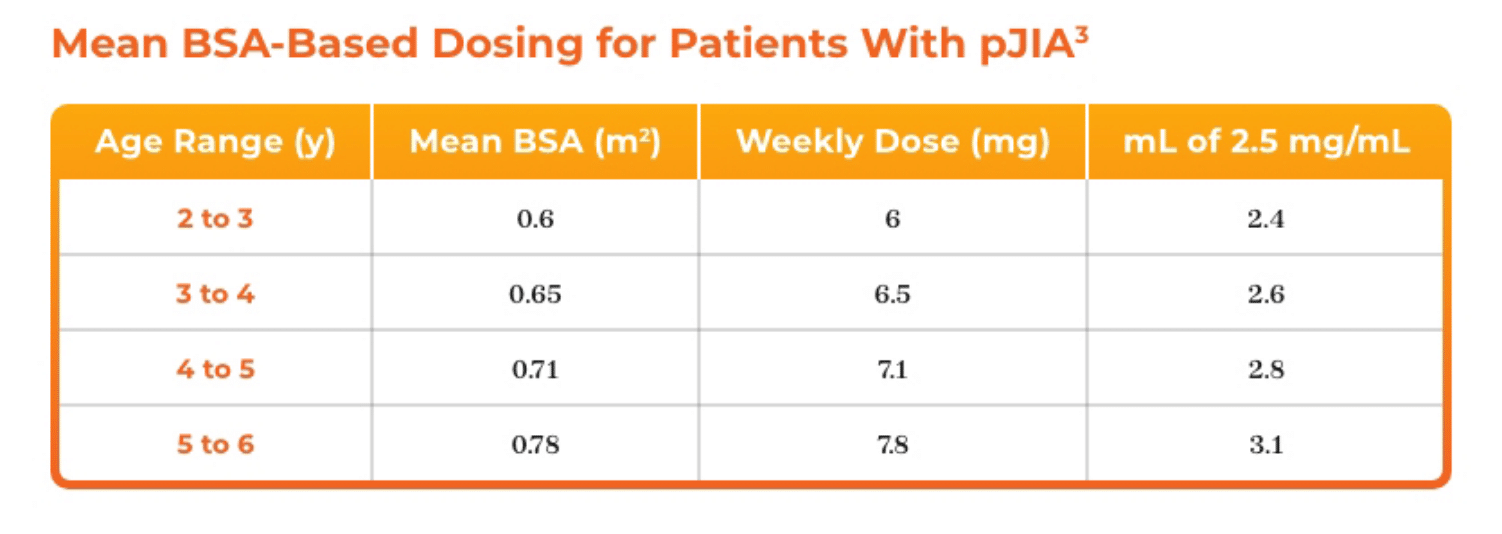
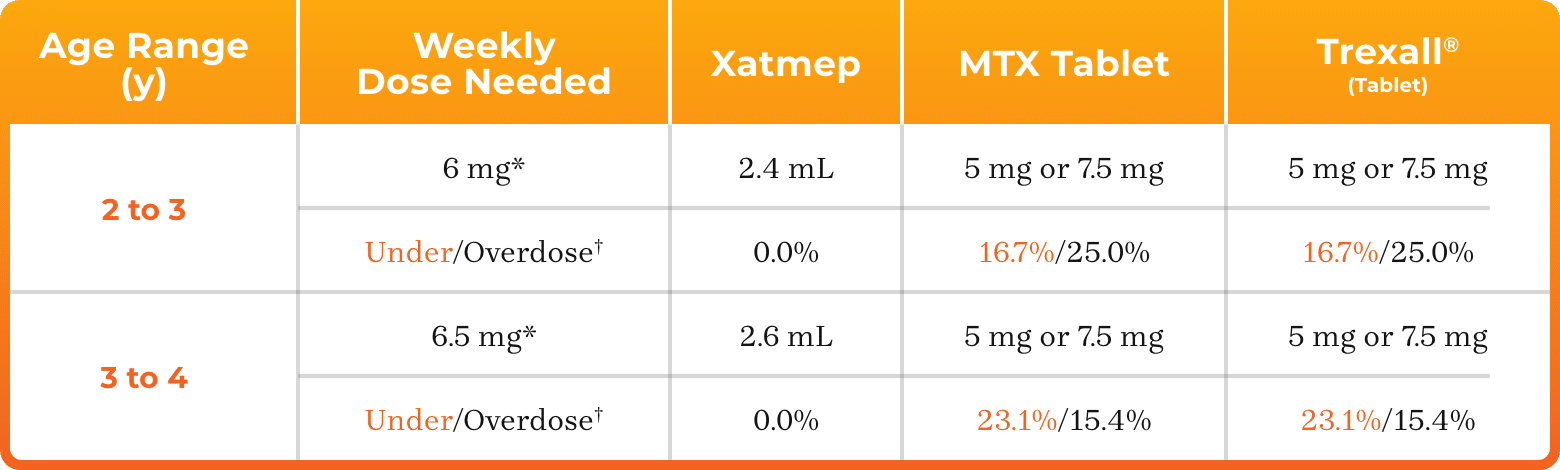
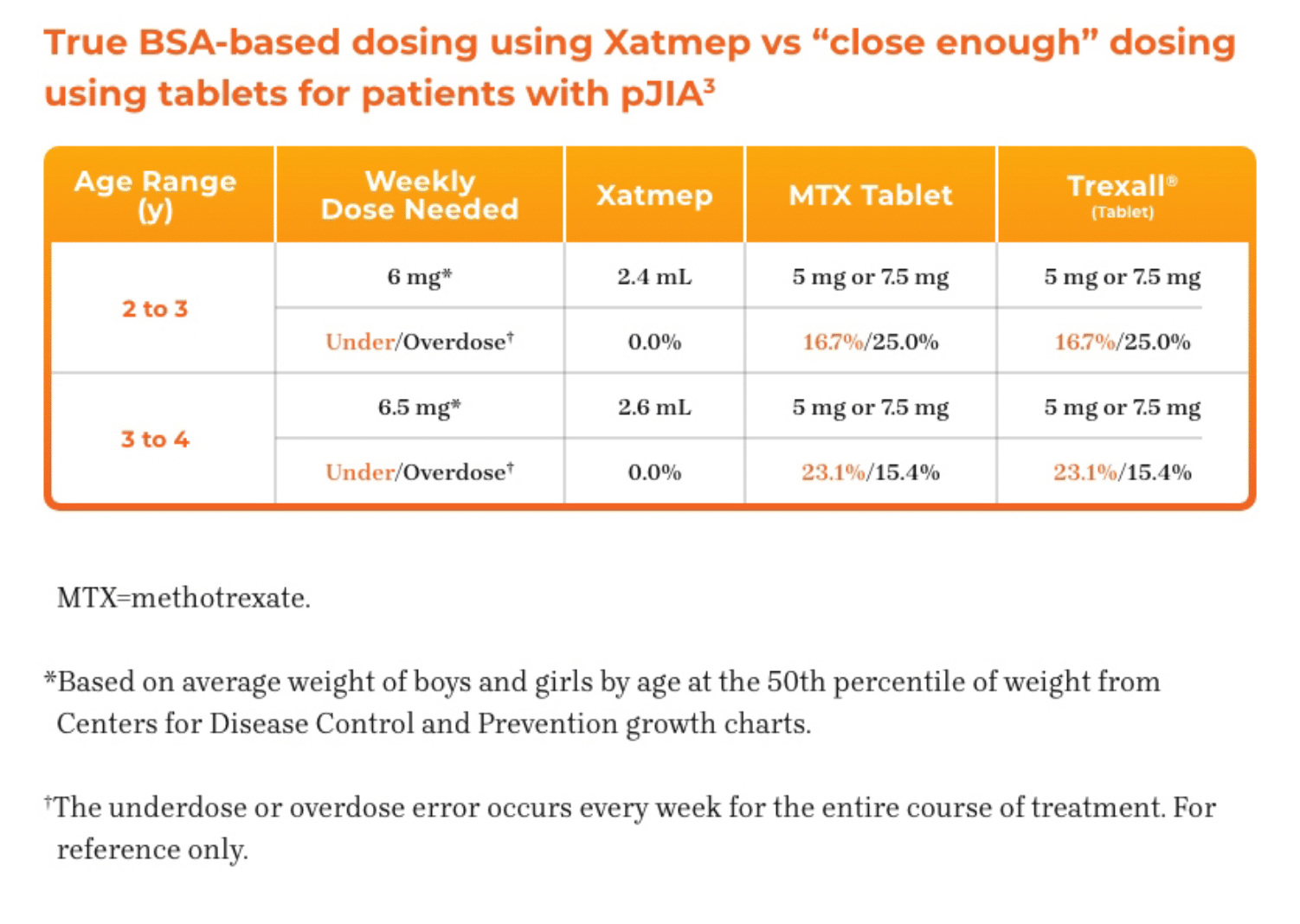
3. Azurity Pharmaceuticals, Inc. Management of Pediatric Patients on Methotrexate Oral Solution. June 2017.
4. Horton TM, Steuber CP. Overview of the treatment of acute lymphoblastic leukemia/lymphoma in children and adolescents. UpToDate.
https://www.uptodate.com/contents/overview-of-the-treatment-of-acute-lymphoblastic-leukemia-lymphoma-in-children-and-adolescents. Updated Nov 29, 2023. Accessed October 31, 2024.